

In practice, it is usually approximated to 1 bar (100,000 Pa).In terms on barometric readings, it is 760 mmHg. Chemical reactions with the container walls C. The atmospheric pressure (1 atm) is 101,325 Pa. A 0.188 g sample of an unknown vapor occupies the flask at 98.7C and pressure of 784 torr.

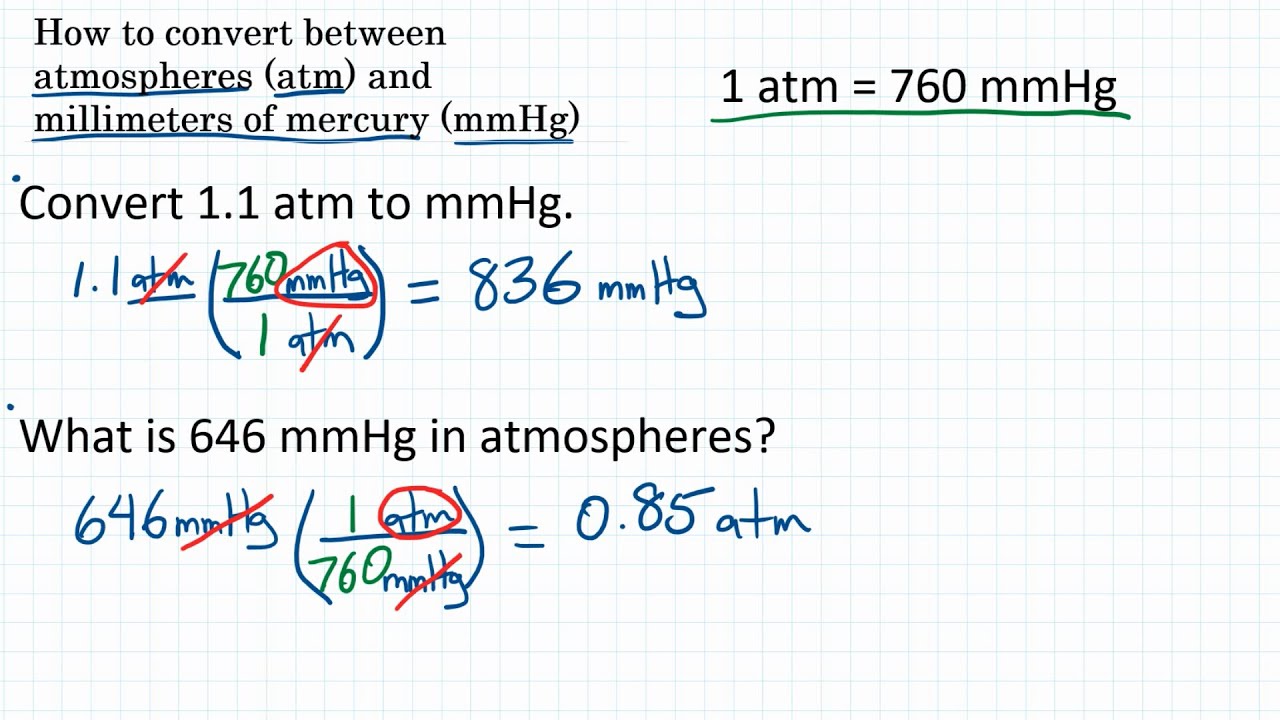
2) A 125 mL Erlenmeyer flask has a measured volume of 152 ml. Necesitan ms mediciones de la densidad de mercurio est disponible. La presin de 1 atm puede tambin ser expresada como: 760 torr 760.001 mmHg, 0 C, ssujeto a revisin. What is the cause of a gas sample exerting pressure on its surroundings, such as a container? A. La atmsfera estndar ( smbolo:atm) es una unidad de presin definida como 101325 Pa (1.01325 bar). What is the final volume, in litres, of the gas at each of the following pressures, if there is no change in temperature and amount of gas? (i) 725 mmHgġ. Produced 880 mL of oxygen were collected over water at a temperature of 25☌ with a totalĪ sample of nitrogen gas has a volume of 50.0 L at a pressure of 760. 1 atm 1.01325 bar atm to bar 1 atm 101.325 kPa atm to kPa 25 pascals atm to pascal 1 atm 0.101325 MPa atm to MPa 1 atm 14.6959 psi atm to psi 1 atm 760 torr (mmHg. what mass of nitrogen is present in the sample?ĥ.8 g of an inorganic metal oxide ore mainly contains 90% of Nickel (III) oxide which decomposes to elemental nickel and oxygen gas. Atm (Atmospheric Pressure) Conversion: An atmosphere (atm) equals to the air pressure at the sea level at a temperature of 15 Celsius. 0.0252 atm (standard atmosphere) 0.026037332 at (technical atmosphere) 19.152 torr 260.373356073 mmH2O (mm of water) 19.152 mmHg (mm of mercury) 0.754015561 inHg (inches of mercury) 0.370338301 psi (pounds per sq. This small difference is negligible for most applications outside metrology. Using the conversion formula above, you will get: Value in torr 65 × 760 49400 torrs. 1 mmHg 1.000000142466321 Torr The difference between one millimeter of mercury and one torr, as well as between one atmosphere (101.325 kPa) and 760 mmHg (101.3250144354 kPa), is less than one part in seven million (or less than 0.000015). The total pressure of the sample is 725mmHg and the partial pressure of argon is 231 mmHg. The formula to convert from mmHg to atm is: atm mmHg ÷ 760 Conversion Example Next, lets look at an example showing the work and calculations that are involved in converting from millimeters of mercury to atmospheres (mmHg to atm). Suppose you want to convert 65 atm into torrs. The standard atmosphere (symbol: atm) is a unit of pressure defined as 101325 Pa (1,01325 bar).
#152 mmhg to atm full
Symbols, abbreviations, or full names for units of length,Īrea, mass, pressure, and other types.A 255mL gas sample contains argon and nitrogen at a temperature of 65 degrees celsius. The pressure of a gas in a container is 152 mm Hg, the equivalent to 0,2 atm. 1.152 kPa (kilopascal) 0.001152 MPa (megapascal) 0.011747131 kgf/cm2 (kg per sq. You can find metric conversion tables for SI units, as wellĪs English units, currency, and other data. Its symbol is Torr.Ĭonversion calculator for all types of measurement units. The torr is a non-SI unit of pressure, named after Evangelista Torricelli. The difference between one millimeter of mercury and one torr, as well as between one atmosphere (101.325 kPa) and 760 mmHg (101.3250144354 kPa), is less than one part in seven million (or less than 0.000015%). The relationship between the torr and the millimeter of mercury is: A gas container is initially at a pressure of 47 mmHg and 77 Kelvin. The decimal form of this fraction is approximately 133.322368421. 6.0 L of gas in a piston at a pressure of 1.0 atm are compressed until the volume is. Therefore, 1 Torr is equal toġ01325/760 Pa. The torr is defined as 1/760 of one standard atmosphere, while the atmosphere is defined as 101325 pascals. The millimeter of mercury by definition is 133.322387415 Pa (13.5951 g/cm3 × 9.80665 m/s2 × 1 mm), which is approximated with known accuracies of density of mercury and standard gravity. Torr to mm Hg, or enter any two units below: Enter two units to convert From: You can do the reverse unit conversion from


 0 kommentar(er)
0 kommentar(er)
